Neuromonitoring Carotid Endarterectomy
In the last post, I talked about an email asking for information about neuromonitoring for carotid endarterectomy surgeries. I told the emailer… “No, problem. Maybe I’ll even put a presentation together that will help you and other people reading this blog.”
Instead of posting the info like I was asked, I went on a rant about using motor evoked potentials for CEA cases.
There was even some further discussion that went on about it on my linkedin account (just scroll down and look for my post about it).
Now, about 3-4 weeks later, I finally put together some presentation material that should help out, or at least give you a head start. It took way longer than I had planned, so if you find this useful, please let me know. Either leave a comment, scroll down and rate it 10 stars, post it somewhere like facebook or linkedin, etc. If enough people find it useful, then I might do some more in the future. If not, then there is no reason for me to put in the time that I did.
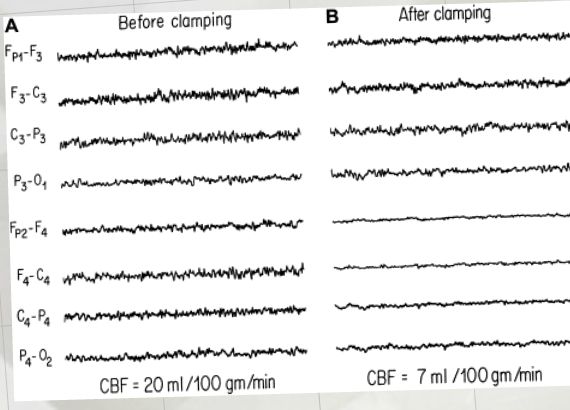
Anyways, the request was to help them make a presentation for vascular surgeon to try to drum up some business. They wanted to let them know the benefits of neuromonitoring carotid endarterectomy procedures. While other modalities like transcranial doppler, cerebral oximetry and stump pressure are commonly used, the request was for EEG and SSEP.
So I broke it down into three sections.
- What is the basis of neuromonitoring for carotid endarterectomy procedures (using EEG and SSEP), and how does it work
- Is it of benefit
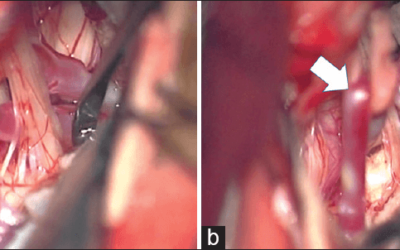
- Examples of how using neuromonitoring during CEA can assist the surgeon that is impossible without it
There’s plenty of other ways to present this, so here’s a couple templates for you to play with.
Here’s a scribd presentation…
Neuromonitoring Carotid Endarterectomy
Here’s a video for monitoring carotid endarterectomy surgeries…
Here’s my prezi for IONM CEA cases…
And here’s the transcripts to the prezi above…
Keep Learning
Here are some related guides and posts that you might enjoy next.
How To Have Deep Dive Neuromonitoring Conversations That Pays Off…
How To Have A Neuromonitoring Discussion One of the reasons for starting this website was to make sure I was part of the neuromonitoring conversation. It was a decision I made early in my career... and I'm glad I did. Hearing the different perspectives and experiences...
Intraoperative EMG: Referential or Bipolar?
Recording Electrodes For EMG in the Operating Room: Referential or Bipolar? If your IONM manager walked into the OR in the middle of your case, took a look at your intraoperative EMG traces and started questioning your setup, could you defend yourself? I try to do...
BAER During MVD Surgery: A New Protocol?
BAER (Brainstem Auditory Evoked Potentials) During Microvascular Decompression Surgery You might remember when I was complaining about using ABR in the operating room and how to adjust the click polarity to help obtain a more reliable BAER. But my first gripe, having...
Bye-Bye Neuromonitoring Forum
Goodbye To The Neuromonitoring Forum One area of the website that I thought had the most potential to be an asset for the IONM community was the neuromonitoring forum. But it has been several months now and it is still a complete ghost town. I'm honestly not too...
EMG Nerve Monitoring During Minimally Invasive Fusion of the Sacroiliac Joint
Minimally Invasive Fusion of the Sacroiliac Joint Using EMG Nerve Monitoring EMG nerve monitoring in lumbar surgery makes up a large percentage of cases monitored every year. Using EMG nerve monitoring during SI joint fusions seems to be less utilized, even though the...
Physical Exam Scope Of Practice For The Surgical Neurophysiologist
SNP's Performing A Physical Exam: Who Should Do It And Who Shouldn't... Before any case is monitored, all pertinent patient history, signs, symptoms, physical exam findings and diagnostics should be gathered, documented and relayed to any oversight physician that may...