Selective Dorsal Rhizotomy Using Intraoperative Neuromonitoring (Guest Post)
(Joe’s notes: This guest post, written by Kristina Young, covers one of those case types where you know you’re going to be part of the surgery today. For a lot of our neuromonitoring cases, no news is good news. But in Selective Dorsal Rhizotomy, we can help the surgeon provide the most appropriate surgical intervention for that individual on the table. I’ve done only a handful of these cases myself, so I reached out to a veteran in the field that has years experience in this case type.)
Neuromonitoring SDR For Kids With Cerebral Palsy
Many of you are probably familiar with a Rhizotomy, but a Selective Dorsal Rhizotomy (SDR) is something a little different. As the name implies, it is “Selective”. IntraOperative Neuromonitoring plays a crucial role in these procedures; in fact, without us in the room, the “Selective” part would be eliminated from the title. I love monitoring these procedures because we really direct the surgeon’s hands. We have a profound impact on his/her actions and therefore the success of the patient.
What is a Selective Dorsal Rhizotomy?
This is a procedure performed on individuals, usually children in particular, who suffer from spasticity as a result of Cerebral Palsy. The theory/goal is that by eliminating the spasticity-causing components of the sensory nerves, the patient will have less tightness and tension in the lower extremities. Post Operatively, following physical therapy, the patient should be able to walk with or without assistance providing they have a high functioning prognosis.
Cerebral Palsy is a general term describing a disorder of permanent, non-progressive movement disorders that cause physical disabilities, primarily in the area of body movement. There are a variety of issues associated with Cerebral Palsy: sensation, proprioception, depth perception, communication, cognition, epilepsy, spasticity, and hypotonia. The symptoms largely correlate with the site of the lesion in the brain. The damage can occur in utero, during childbirth, or after birth up to age three. The location of the lesions can be pyramidal or extrapyramidal.
Cerebral Palsy
Let’s discuss the two causes of Cerebral Palsy that are usually correlated with spasticity; this diagnosis is necessary for patients to be candidates for successful SDRs.
The first type of Cerebral Palsy that can result in a child with spasticity is caused by a PVL- Periventricular Leukomalacia. A PVL denotes the patient has had damage to the white matter of the brain; there is early cell death in the white matter, leaving holes (lateral ventricles) that fill with fluid. This occurs most often due to an infection while the baby is still in utero (26-34 weeks gestation) or it can occur in babies born prematurely (prior to 32 weeks with a birth weight of fewer than 3.3 lbs.). These patients typically present with spastic diplegia or spastic quadriplegia which is spasticity from either the lower limbs or all four limbs respectively.
The second cause of Cerebral Palsy that can result in a child with spasticity is an IVH- Intraventricular Hemorrhage. An IVH, simply describing it, is bleeding inside or around the ventricles of the brain. This can be from venous or arterial damage and the severity is dependent on the location of the bleed. Arterial bleeds tend to be more severe than venous bleeds due to the fact that arteries are carrying oxygenated blood and are harder to control.
This is most prevalent in babies that are born prematurely, prior to 32 weeks with a birth weight of fewer than 3.3 lbs. These patients typically present with spastic hemiplegia meaning one side of the body’s limbs are affected.
The two causes of Cerebral Palsy listed above affect the patient’s pyramidal tracts of the brain (pyramidal neurons in the cerebral cortex down to the brainstem) which is the pathway that we know begins our corticospinal tract. Other causes/types of Cerebral Palsy tend to affect the extrapyramidal tracts of the brain (basal ganglia, thalamus, or cerebellum). These patients do not have a diagnosis of spasticity and would therefore not be candidates for a successful selective dorsal rhizotomy.
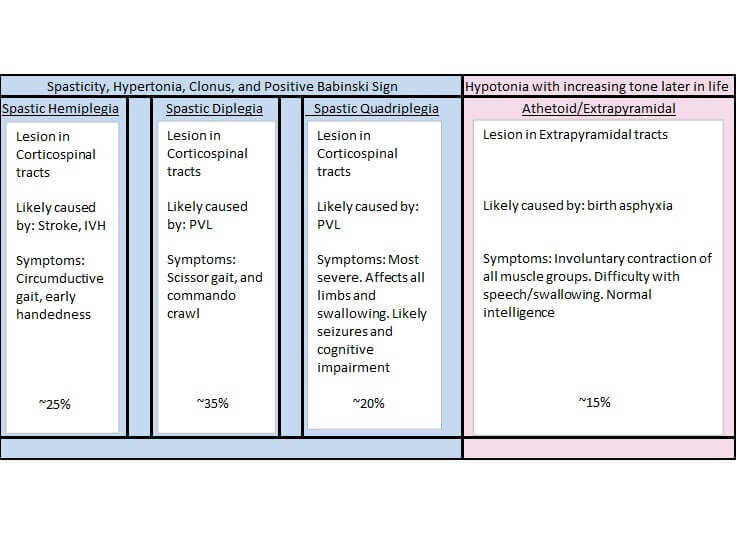
Figure A is a chart showing the different types and causes of Cerebral Palsy along with possible symptoms.
Spasticity In Cerebral Palsy
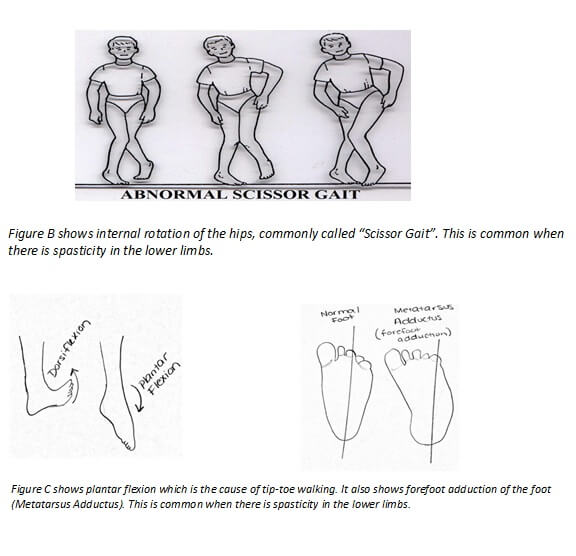
The characteristics of spasticity include resistance to movement, decreased the range of motion, brisk reflexes, and clonus. In the lower limbs, it is common to see an internal rotation of the hips with plantar flexion and forefoot adduction. As a result of monitoring many SDRs in the past, I have noticed that children with internal rotation of the hips (scissor gait) seem to have more sensitive nerve rootlets at the higher levels (L1-L2); where those children with extreme forefoot adduction (metatarsus adductus), who are tip-toe walkers, seem to have more severe responses from lower levels (L5-S2).
A common diagnostic tool used to determine if a patient has an upper motor neuron lesion is the Babinski test.
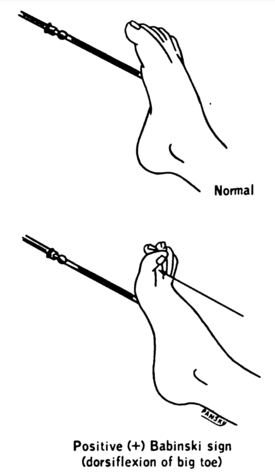
Figure D demonstrates a Babinski test. The lateral side of the foot is stroked by a sharp metal object. The resulting response from the foot is considered normal if the toes curl forward, but abnormal if the toes fan up and outward. An abnormal response is indicative of an upper motor neuron lesion.
Localizing The Lesion
You also may be asking yourself, why do these patients have spasticity in the limbs, especially from the lower extremities, if the lesion is in the brain? While there isn’t a perfect answer to this question, it is found that local inhibitory networks in the spinal cord are reduced in these patients. This renders the system hyperexcitable. When sensory input enters the spinal cord of a spastic patient, suprasegmental and contralateral connections go uninhibited leading to the activation of alpha motor neurons, which are normally not excited by these sensory inputs. Sensory stimulation of isolated afferents on one side can even result in aberrant muscle activity from the contralateral limb!
How Did Selective Dorsal Rhizotomy Start?
It’s time now to discuss the procedure- the Selective Dorsal Rhizotomy. The idea of performing SDRs to relieve symptoms of spasticity is not a novel idea, but it has come a long way since its origin. The idea originated in 1898 when C.S Sherrington studied the relief from muscle spasticity in decerebrate cats after sectioning dorsal roots. In 1913 the first surgeries were performed on humans by Otfrid Foerster. He performed posterior root rhizotomies but, patients typically had poor outcomes and suffered from extreme weakness in the lower extremities. After this, rhizotomies took a hiatus for about 50 years. In 1978, Fasano of Italy began experimenting with the “Selective” idea. Following Fasano, W. Peacock of South Africa developed the idea to expose the cauda equina instead of the spinal cord. In 1986, Dr. Peacock moved to LA and began to campaign the SDR to relieve Cerebral Palsy patients of spasticity. Now, many surgeons are performing selective dorsal rhzotomies throughout the world.
A selective dorsal rhizotomy is performed in the following way. The IntraOperative Neuromonitorist should place pediatric or adult sized subdermal needles in the following muscles utilizing bipolar pairs: Iliopsoas (L1), Adductor Longus (L2, L3, L4), Vastus Lateralis (L2, L3, L4), Bicep Femoris (L5, S1), Tibialis Anterior (L4, L5), Gastrocnemius (S1, S2), Adductor Hallucis (S1, S2), External Anal Sphincter (S2, S3, S4). Test parameters should be as follows:
EMG parameters:
- Low-frequency filter 10 Hz
- High-frequency filter 1-3 kHz
- Display gain 50-200 uV/div
Stimulation settings (50 Hz train):
- 10 ms duration, trains of 50 pulses/sec for 1 sec.
- Timebase of 200 ms/div for 10 divisions. This allows for observation of pre-stimulus activity (200 ms), stimulus train (1,000ms), and post-train activity (800 ms)
- Use a 200 mS trigger delay

Figure E gives an example of the window layout for 50Hz t-EMG showing the recording window composed of: Pre-Stimulus of 200 mS, the stimulus of 1,000 mS, and the post-stimulus of 800 mS.
Anesthesia During Selective Dorsal Rhizotomy
As with most IntraOperative cases, we do have some requests for the anesthesia team. For EMG only, we are primarily concerned with neuromuscular blocking agents. It is important that the patient maintains a full Train of Four to ensure true results from testing. Inhalational agents such as Desflurane, Sevoflurane, and Isoflurane can be used, as well as Propofol and narcotics. It is desirable to keep concentrations of inhalational agents at or below 1 MAC.
Selective Dorsal Rhizotomy Surgery
Once intubated and prepared by the neuromonitoring team, the patient will be positioned in the prone position. Bear in mind, the patient is usually pediatric between the ages of 2 and 10. An X-ray will be utilized to approximate the location of T12-L2. An incision will be made. A laminectomy will take place (the levels vary according to surgeon preference, but most prefer to do a limited laminectomy right below the conus medullaris). An ultrasound is then used to confirm the location of the conus medullaris.
Figure F
Figure F shows an ultrasound of the conus medullaris confirming the level at which the surgeon will proceed to perform the laminectomy.
Once the conus medullaris has been identified, the dura will be opened revealing the roots of the cauda equina. A microscope will now be utilized. The surgeon will attempt to separate the dorsal roots (sensory) from the ventral roots (motor) via visual inspection and known anatomical landmarks. Single pulse triggered EMG should be utilized to verify any roots that pose the surgeon with any uncertainty. Stimulation of a direct motor root should evoke a response in the corresponding muscle group with an intensity as low as .05 mA. Sensory roots typically fire anywhere from .5-4 mA. Once verified, the dorsal and ventral roots will be separated by silastic pads. At this point, IntraOperative Neuromonitoring takes the spotlight. For the rest of the procedure, we will communicate continuously with the surgeon.
The surgeon is provided with two probes that make an electrical circuit- one probe is the anode and one is the cathode. The surgeon will then isolate a sensory root (usually starting at L1 or L2 provided this is below the conus medullaris). The surgeon will divide the root into rootlets with these two probes that function to stimulate and divide. The surgeon typically divides each individual root into 5-8 rootlets. Once the division has taken place, each rootlet is stimulated to obtain a threshold (just like you do when you do pedicle screw testing). It is important to note the orientation of the probes; you want the cathode to be positioned rostral to the anode. Remember, the desired direction of the current is towards the spinal cord.
Figure G

Figure G shows the probes and connector wires that the surgeon uses to separate and then stimulate the sensory rootlets.
Once a threshold is obtained, the same rootlet will be stimulated with a 50 Hz train stimuli at that same intensity. The resulting t-EMG patterns will be rated on a scale of 0-4 and the surgeon will either cut the rootlet or spare it. It is important that the IntraOperative Neuromonitorist document the fate of each and every nerve rootlet. Most surgeons prefer to lesion about 50% of the rootlets in every case. Our role is to determine which rootlets are more responsible for the patient’s spasticity and which are not.
Scoring
There are a few different scoring methods to determine the fate of sensory rootlets. I will discuss the method I often use, which is the one I believe is most popular among surgeons. I will describe the T.S. Park, P.E. Gaffney, B.A. Kaufman, and M.C. Molleston method. A summary of the grading scale is shown in the figure below.
Figure H
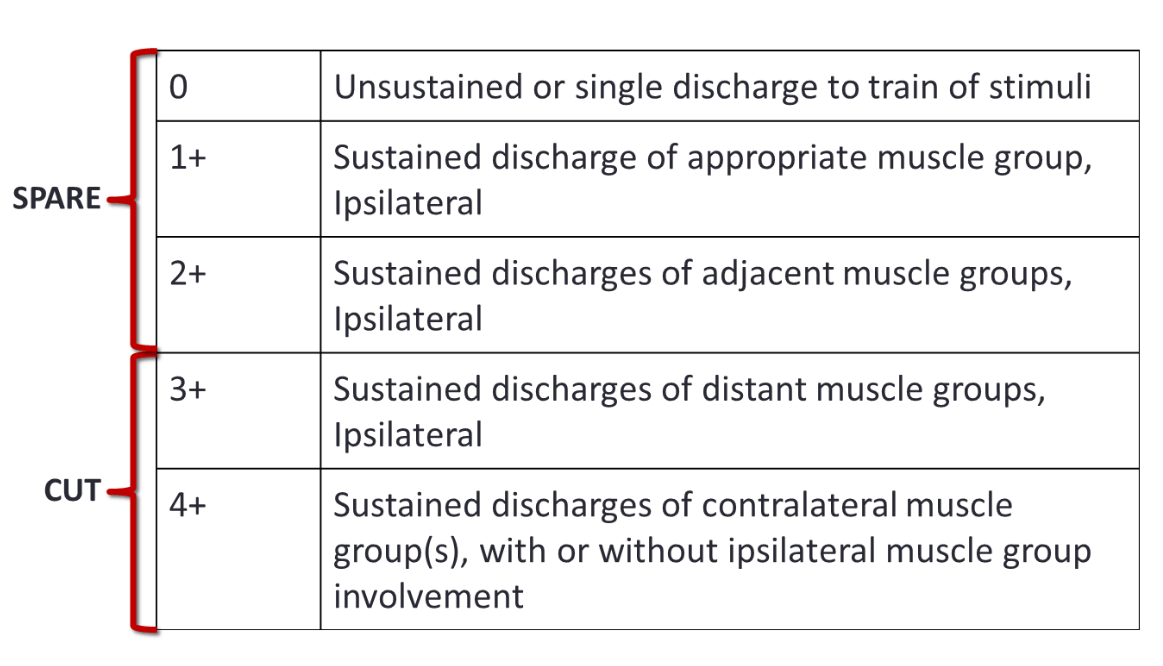
Figure H summarizes the patterns of 50 Hz t-EMG and the corresponding grading scale.
The surgeon continues to test each nerve rootlet in the same fashion from L1/2-S1/2 (this depends on how conservative or radical a surgeon is). The surgeon I work with decides that he is complete when the single pulse t-EMG threshold yields responses primarily occurring in the External Anal Sphincter.
Now, something to note is that typically a surgeon is making a very educated guess about which nerve root he is working with at any given time if he performs a one level laminectomy. Some surgeons will perform a large laminectomy (L1-S1) to visualize each nerve root as it exits its foramina. This latter method provides IntraOperative Neuromonitorists with better information to grade rootlets; however, it has been found that the limited, smaller laminectomy is better for growing pediatric patients. I think the limited laminectomy is much more common today than a full open laminectomy.
Once all of the dorsal nerve roots have been divided, tested, and cut and/or spared, the procedure is complete. As I mentioned earlier, the surgeon usually prefers to cut around 50% of all dorsal rootlets on average. The dura will then be closed, the skin closed, and the patient will be taken to recovery.
SDR Example Traces
Feedback from the surgeon I work with has been very positive. He has and will continue to utilize Neuromonitoring for all SDRs. I have included some of my SDR data below.
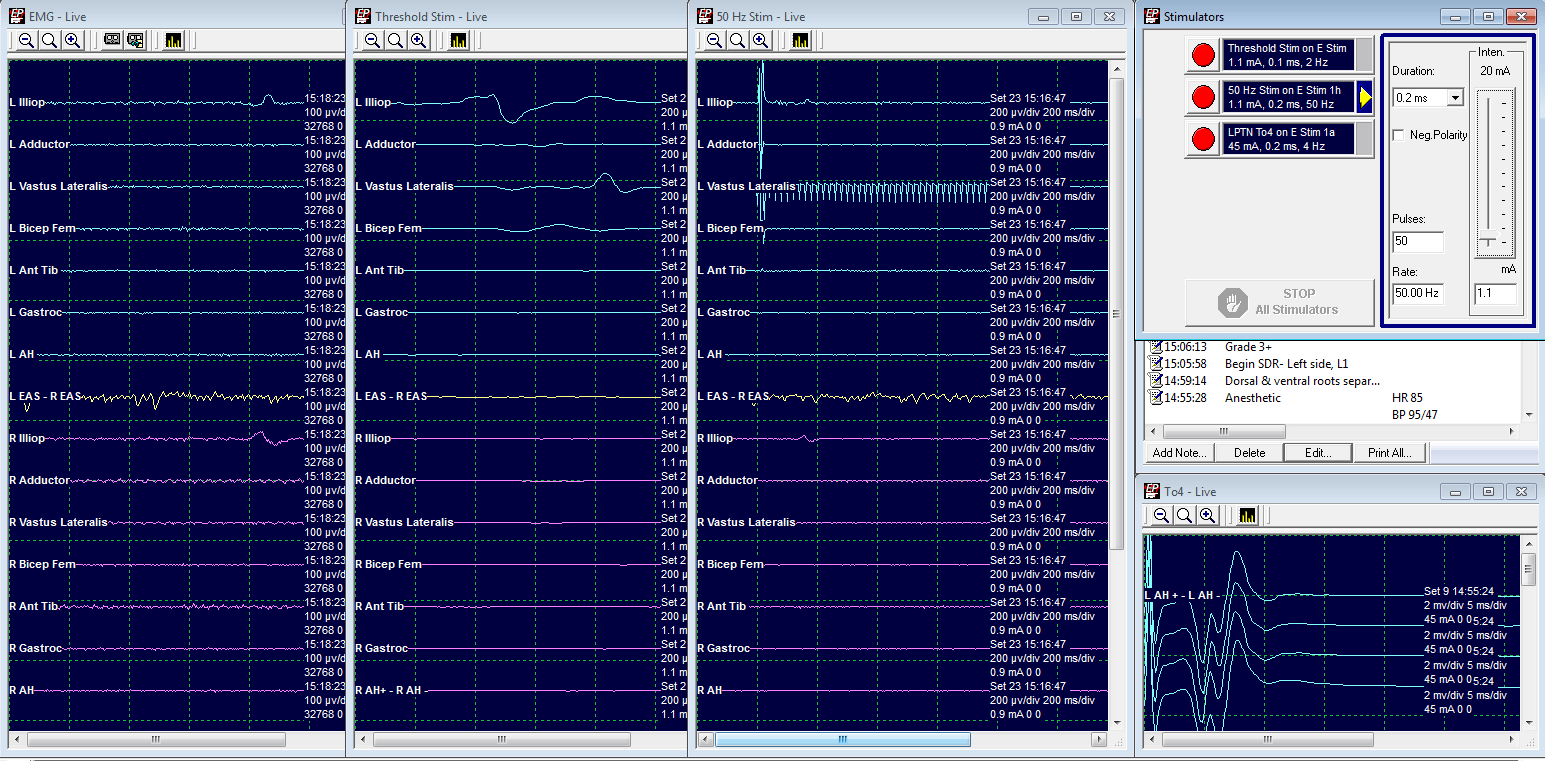
Grade 1: Spared
LL2 Threshold at 9. mA.
50 Hz train at .9 mA elicits a response in L Vastus Lateralis only.
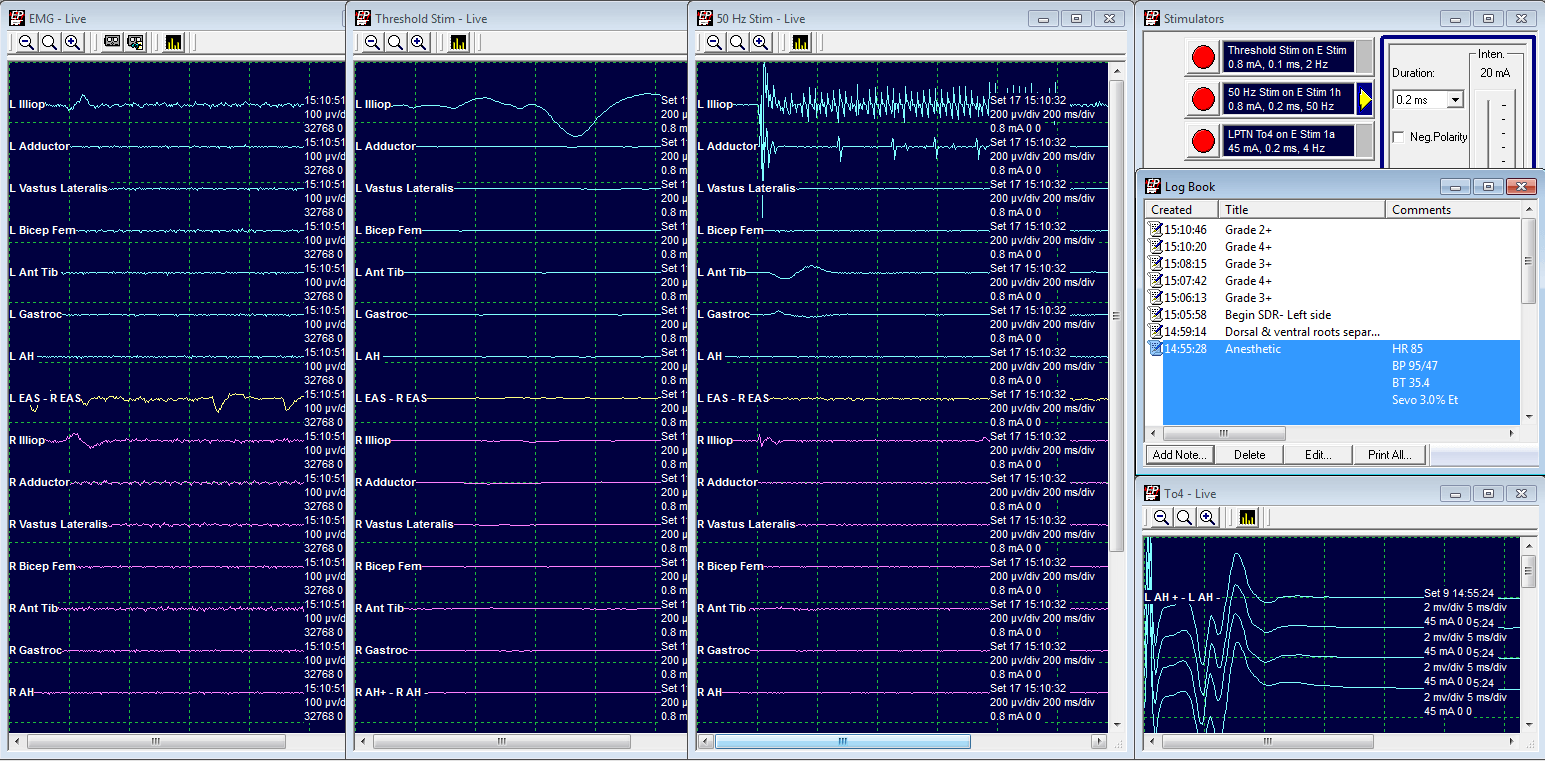
Grade 2: Spared
LL2 Threshold at .8 mA
50 Hz train at .8 mA elicits a response in L Iliopsoas and L Adductor.
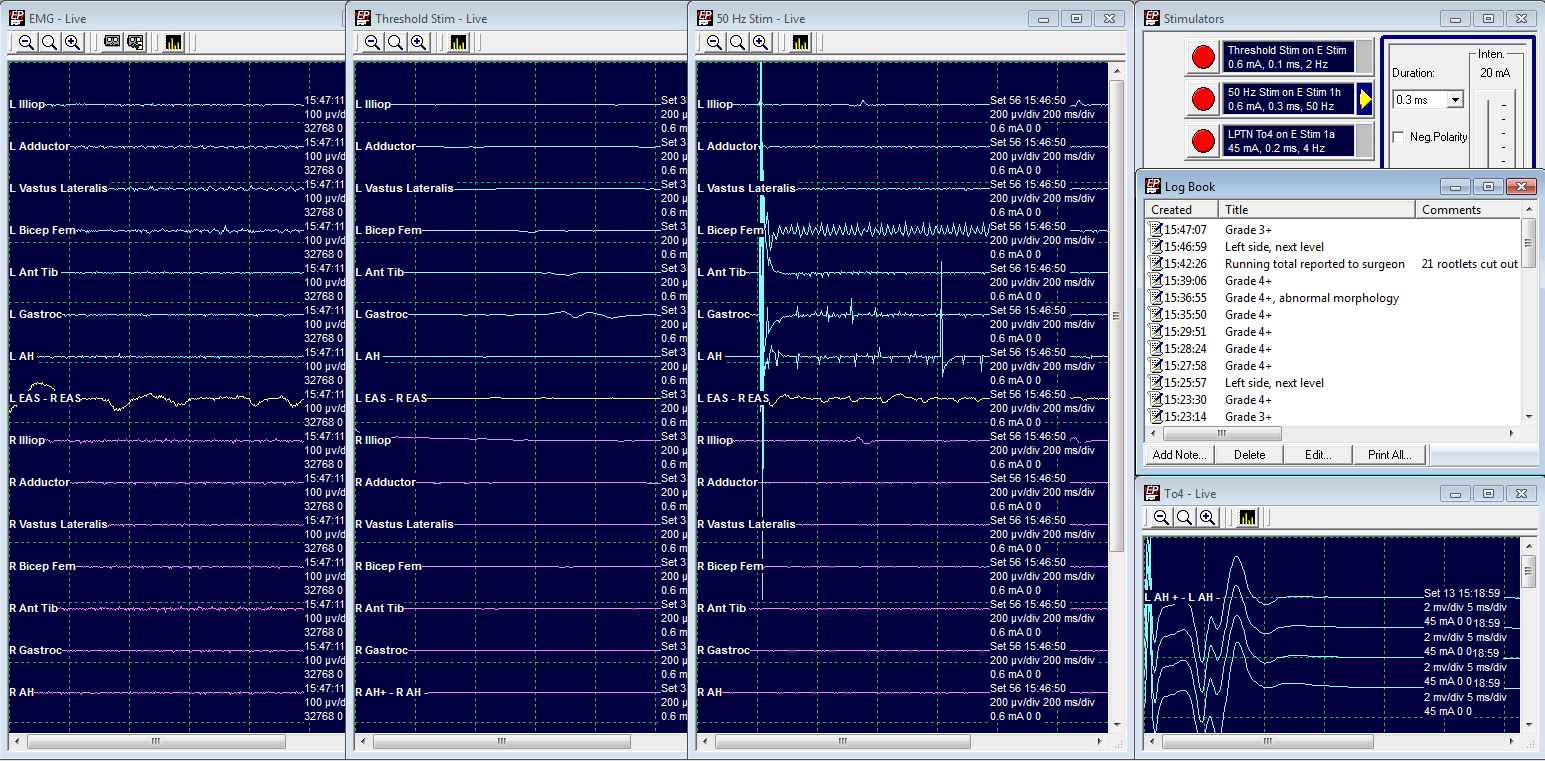
Grade 3: Cut
LL5 Threshold at .6 mA
50 Hz train at .6 mA elicits a response in L Bicep Femoris, L Gastrocnemius, and L Adductor Hallucis.
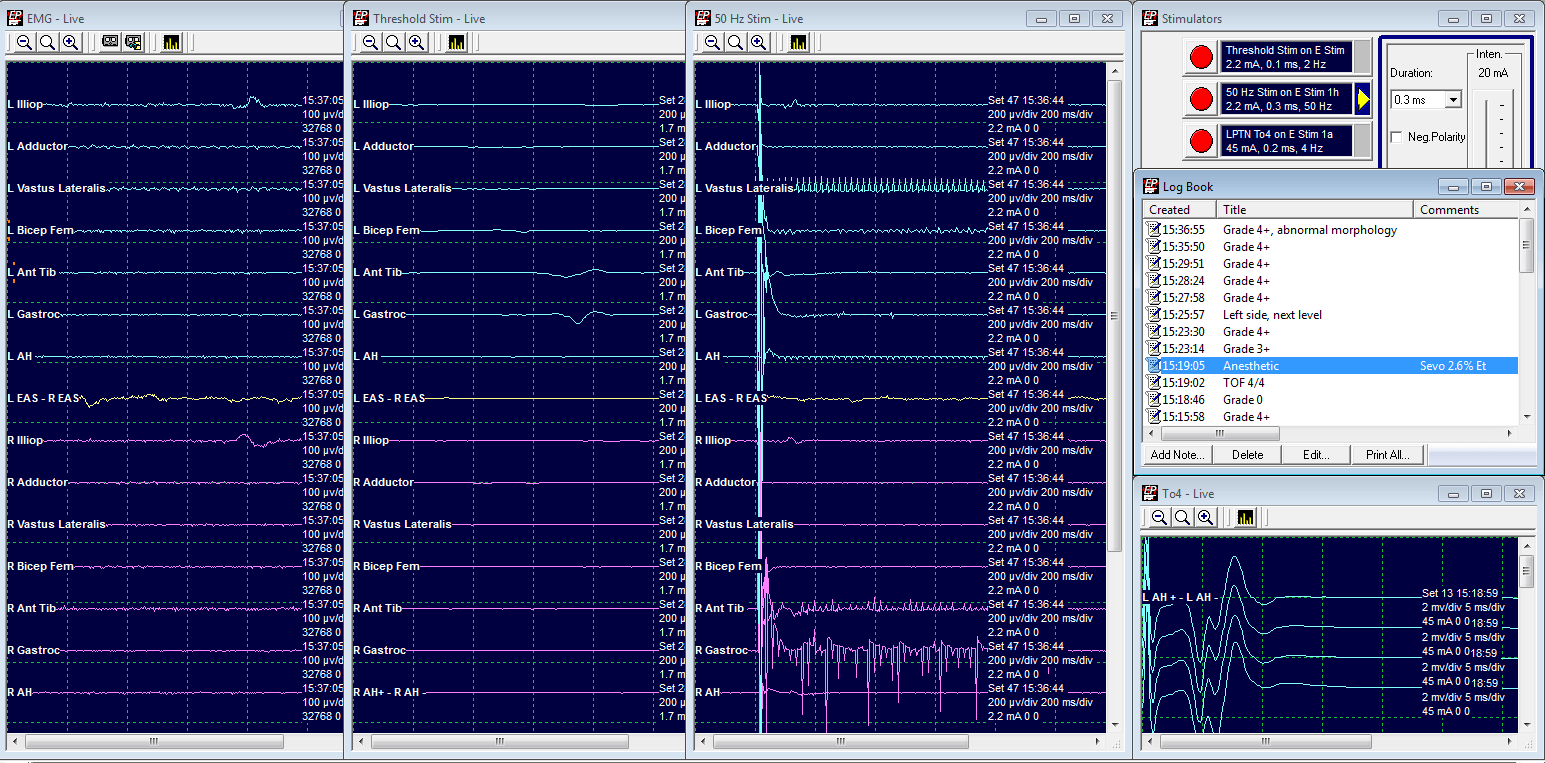
Grade 4: Cut
LL5 Threshold at 2.2 mA
50 Hz train at 2.2 mA elicits a response from both ipsilateral and contralateral limbs.
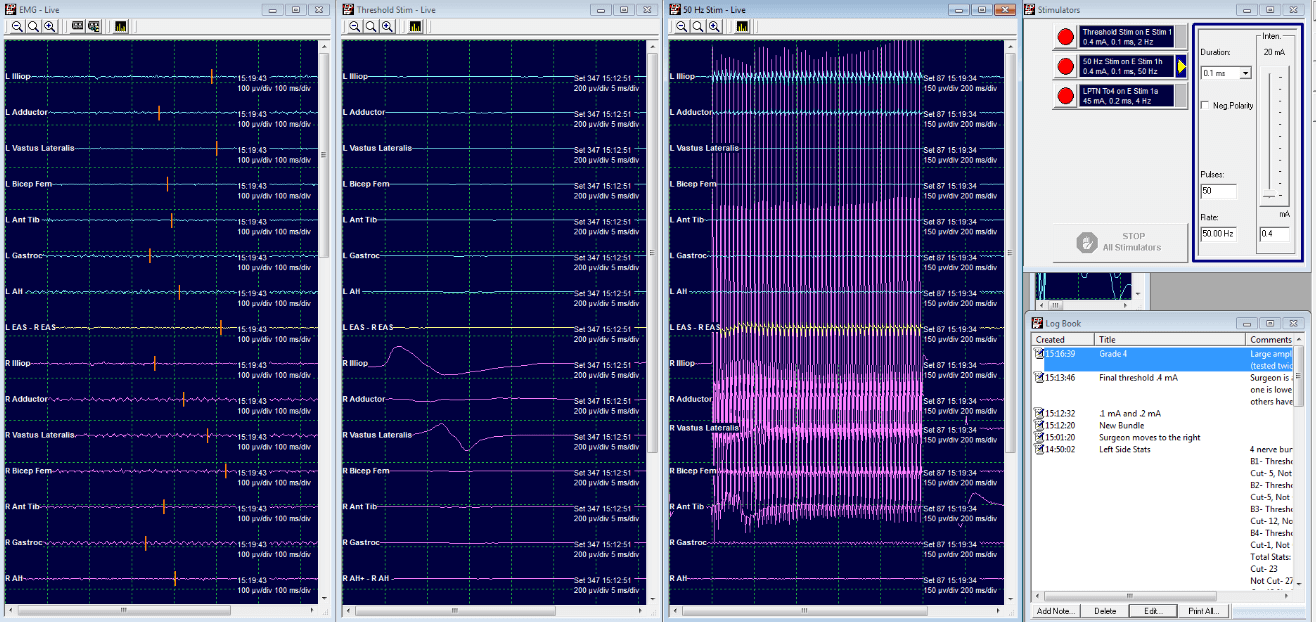
Grade 4: Cut
LL2 Threshold at .4 mA
50 Hz train at .4 mA elicits a large response from both ipsilateral and contralateral limbs.
References:
C.D. Yingling: Selective Dorsal Rhizotomy
T.S. Park, P.E. Gaffney, B.A.Kaufman, M.C. Molleston: Selective Lumbosacral Dorsal Rhizotomy Immediately Caudal to the Conus Medullaris for Cerebral Palsy Spasticity
W.J. Peacock, L.A. Staudt, M.R. Nuwer: A Neurosurgical Approach to Spasticity: Selective Posterior Rhizotomy

Kristina Young, B.S., CNIM
Director of Training in Surgical Neurophysiology
Coronavirus: Big Ideas To Carry Over to Intraoperative Neuromonitoring…
NOTE: At the time of this writing, we're on the front side of the Coronavirus outbreak. I prefer to only write timeless over timely material, but there are certain moments in history that affects our IONM community. This one feels really, really different. Our...
The Game of Chicken: How Managers Model the Inevitability of Conflict…
There's a quote that goes something like this: "what's the definition of conflict? Two people in the same zip code..." Why? Because we're programmed for it. We prioritize survival and reproduction over everything else. Contrast is an edge in survival and selecting a...
Beware of the Big Hats – The Incentives Are Off…
I was asked to give a talk this year at an ASNM meeting in Tampa. The topics requested were all in the area of "things most people are afraid to do, so not spine or craniotomies." That left the door open and I decided to focus more on helping solve the problem of why...
What’s Next? How About Worrying Less About Being Better?
I have to admit, the first decade of my IONM career aimed at better. Not stick-my-nose-in-the-air better, just better than I was yesterday. Better is hard -- painful at times. You sometimes have to work hard on things when you'd rather not. There're expectations laid...
The Surgical Neurophysiologist’s Nemesis (and Ally): Volatility.
Fortunes are made and lost on the back of volatility. If you were a day trader with investment velocity as a strategy, you'd pay less attention to the megacorps in a slow-growth sector. The seas are too calm. There are no white caps to identify before the break in a...
False Dichotomy in the OR: It Is or It Ain’t…
If you're in America (this might be happening elsewhere, too), the world we live in feels a little different. Information flow was previously more limited and therefore controlled: the general consensus was familiar and comfortable. Opinions and understandings were a...