Double-Train MEP On A Comeback Kick
Using transcranial electric motor evoked potentials in the operating room has become routine practice for spinal cord monitoring. Recent improvements in the ability to record tcMEP have resulted in increased use during other procedures, like brain surgery, vascular surgery and peripheral nerve monitoring. One of the techniques utilized in the optimization of transcranial motor evoked potentials is double-train MEP.
Some find double-train MEP to be helpful in cases where tcMEP are difficult to elicit using single, multi-pulsed cortical stimulation. But it isn’t always the best way to elicit a response, and only utilized if other optimization strategies fail to reproduce reliable compound muscle action potentials.
Others think double-train MEP should be the new standard and feel reprogramming all single, multi-pulsed MEP templates to double-train tcMEP templates should have been done years ago.
Here I take on the two point of views, just as I would if I was preparing for the D.ABNM exam, to answer the question: “should we be using double-train MEP on all our cases?”
Double-Train tcMEP
I'm Not Sold On Double-Train Motor Evoked Potentials
I Find Good Reason To Start Each Case With Double-Train tcMEP

“Can you go over the difference in Double-train MEP vs single train MEP?”
Single vs Double-Train MEP
Double-train tcMEP consist of running an initial number of motor evoked pulses, followed by a brief break. The initial set of pulses usually consists of 1-4 pulses, with an interstimulus interval, or ISI, appropriate to the area of most concern. These are referred to as the conditioning stimuli. The brief break in stimulation referred to as the inter-train interval, or ITI, separates the first train of pulses from the second. The second train of pulses usually consists of 4-7 pulses. Again, the interstimulus interval can vary depending on the CMAPs collected. This second set of stimuli is referred to as the test stimuli.
Why Consider A Double-Train Transcranial Electrical Stimulation?
tcMEP do not summate potentials solely by monosynaptic transmissions, such as seen in D-wave monitoring. When stimulating transcranially and recording activity over a muscle, I-waves also play a critical role in bringing the alpha motor neurons to summation through indirect pathways via interneurons. It does this by depolarizing the alpha motor neurons and increasing their excitability, allowing for subsequent stimuli to have a better chance of again causing an action potential.
This was observed as a necessity for transcranial electrical motor evoked potentials in the operating room, where the inhibitory effects of anesthetics on the alpha motor neurons prohibited summation of those neurons and collection of a compound muscle action potential.
In order to overcome the suppressive effects, it was recommended to run a train of stimulation with multiple pulses. These rapid firing pulses, usually using a rate of 200-1000 pulses per second (for my Axon users) or 1-5 ms for the interstimulus interval (for my Cadwell users) took advantage of temporal and spatial summation and allowed collection of a compound muscle action potential under anesthesia.
And as the neuron directly summates on the alpha motor neuron (D-wave), it also has effects on surrounding neurons within its network at both the cortical and spinal cord level. Not only does that allow for other neurons to possibly fire (I-waves), but it also increases the resting membrane potential of those neuronal pools as well.
So after we run a set of tcMEP for a train of say 7 pulses, we get 7 D-waves and (hopefully) a lot more I-waves to help activate a response. We are also left over with some pretty excitable neurons.
These rapidly firing pulses are right at the cusp or very close to the time elapsed during the relative refractory period after a neuron is brought to the threshold (around 1-2 ms). Some of the neurons brought to threshold might not have been able to create a subsequent response and will have to wait for next pulse or later I-wave potentials in order to fire again. If not, then it just sits closer to the threshold for a little bit and then retreats back to its level of resting membrane potential.
That led to some researchers asking the following question…

“What if we waited for a certain amount of time after an initial stimulation to allow the resting membrane potentials of a large population of cortical and spinal neurons to become more positive… and then hit them with some more pulses?”
The Conditioning Stimuli
By doing a conditioning stimulation, you’re priming the pump for a certain area. You’re allowing a greater area of neurons to become excited and sit closer to the threshold. In theory, you would have more neurons ready to fire should a new wave of pulses be delivered. So when the second train of pulses delivered, it activates a separate area of neurons that has overlap with the conditioned stimuli. This overlapping area, if timed correctly, is where additional facilitation can occur.
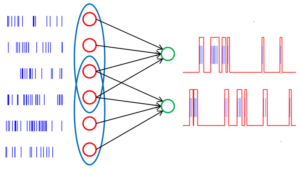
How much output is needed to prime the pump?
This is important to consider when selecting a number of pulses in the conditioning response. Should you choose a train of 1, you are most likely relying on direct, monosynaptic excitation as your mode of increasing alpha motor neuron excitability (although indirect pathways may be activated with enough power output). Should you choose to use multiple trains with an appropriate interstimulus interval, then you are taking advantage of direct and indirect means of exciting the cortical and spinal neurons.
But we have some restrictions we need to consider when choosing the number of pulses for the conditioning response.
First – There are some time constraints to consider. If we’re looking to record over a head or proximal muscle, then we need to take into account the number of trains + the rate at which we stimulate. To be honest, if you are staying within the recommended tcMEP parameters, you will not have to worry about this. It may become a problem with a longer ISI.
Second – We need to make sure that we do not exceed the limitations of the equipment for output delivery. On the Cadwell, the machine will allow you to adjust the settings outside of what it will actually output. Be aware that once you hit those limitations, you are no longer changing the output.
Third – We need to find the number of pulses that brings a large group of neurons close to the threshold, but not actually bring it to the threshold. This is a conditioned response and there seems to be an inverse relationship between amplitude size of the conditioned stimuli and test stimuli. If we are seeing a CMAP on our conditioned stimuli, then we may be causing excitation of IPSP. You will find yourself better served to allow for more trains to be used on the test stimuli.
The tcMEP Intertrain Interval
The next major consideration is the intertrain interval. As it turns out, there are certain times where the overall excitation is greater than other times, and it is not a straight declining line.
As described by (Journee, 2007), there are times where the test stimuli will have maximal amplitudes for motor evoked potentials, or where excitation is the greatest, and times where there are minimal amplitudes, or where inhibition is the greatest, when compared to the same test subject without any conditioned stimuli.
So what might be causing the periods of decreased amplitude during double-train MEPs?
- It is proposed that many spinal interneurons are acted on through oligo- and polysynaptic pathways that can lead to the summation of both excitatory postsynaptic potentials or inhibitory postsynaptic potentials.
- After the conditioned response, the amount of neurotransmitter available at the spinal cord level might be insufficient.
- After the conditioned response, there might be some time period were the neurons demonstrates some refractive properties and will not allow the threshold to be reached again for a period of time.
- Cortical inhibitory interneurons acting on pyramidal cells causing a “silence period” of muscle activity.
- An intensity too great during the conditioning response may evoke a motor response, which also may evoke Renshaw inhibition at the alpha motor neurons following the response. This may effect short durations used on intertrain intervals than longer durations.
As you can see from their studies, a short intertrain interval and long intertrain interval recorded signals that were greater than the amplitude of a single, multi-pulsed train. But in the middle intertrain interval, the signal was less than the single, multi-pulsed response.
Practical use of double-train motor evoked potentials
At this point in time, I think we have some decent guidelines to follow, but nothing set in stone. Additional cases are needed to help validate any recommendations.
That being said, it looks as if double-train motor evoked potentials may help improve signal acquisition, further optimizing responses and allowing for the collection of data in patients with difficult to obtain signals (diabetes, peripheral nerve injury, myelopathic, etc.). Starting points should be as follows:
- Use a lower stimuli intensity for the conditioning response. Too high of an intensity of the conditioning response may lead to Renshaw inhibition at the spinal cord level and a decrease in amplitudes. Amplitude will increase in tandem up to around 200V, where the conditioning response amplitude will continue to grow while the test response will start to attenuate. A conditioning response of 175-250 V seems to be the sweet spot.
- Use only a couple of conditioning pulse and more test stimuli. It doesn’t take a lot of pulses to prime the alpha motor neurons, but you can still take advantage of spatial and temporal summation of a multipulse signal for the test stimuli. 4 pulses + 4 pulses do not seem to work as well as 2 or 3 pulses + 6 or 7 pulses.
- Use either a short duration intertrain (15-40 ms) or a long intertrain interval (around 200-500 ms). A middle duration intertrain interval (40-100ms) seems to have inhibitory effects and a lower amplitude compared to single, multi-pulsed motor evoked potentials.
- Be cautious of even greater patient movement. We are looking to ramp up the alpha motor horn cells, which will affect proximal muscles as well as distal. This may cause the patient to “jump” more.
My personal experience…
I’ve tried the double-train motor evoked potential on both the Axon and Cadwell. I personally did not see much advantage to using the double train vs a high number of pulses using a single train technique.
Besides trying to improve my own technique, the initial reason I wanted to test out this technique was to see if there were any non-physiological advantages to using it. Mainly speaking… will my machine give me more flexibility?
I wondered if the increasing amount of time between trains would reduce the amount of charge allow me to go a little higher if needed without any additional risk of burns or seizure activity. Since the system will still regulate you on the number of total pulses and intensity delivered, there was no additional benefit there.

But it was still worth trying it out to see if there are physiologic benefits to delivering the motor evoked potentials in this manner.
From my (admittedly limited) experience there was not.
Here are the parameters that I used:
The Champion: Single train = 9 pulses, ISI = 2 ms, pulse duration = 50 us, intensity = 300V
Vs.
The Challenger: Double train = 2 + 7 pulses, ISI = 2 ms, intertrain interval 15 and 250, pulse duration = 50 us, intensity = 300V
The first thing you might see is that I stimulated with 300V, which is higher than recommended elsewhere. On all of the cases monitored, anesthesia was at 0.6-0.8 MAC of an inhalational agent (Desflurane or Sevoflurane) and a remifentanil drip. I did try to dip down to 200V, but the results were way worse at that point. The majority of the cases I tried them on were cases that I was able to get a reliable response with a single train delivery, allowing me additional time before the case started to test out the double-train MEP. I also tried it on a couple of cases with poor, but monitorable recordings. I didn’t observe any benefit. I’ve since then stopped tinkering around with double-train motor evoked potentials, but will still give it a shot if all my other optimization tricks aren’t working.
What about you? Are your results different than mine? Are you so sold on double-train MEP that you switched all your programs to default to it from the start, or do you use it as an optimization strategy?

Joe Hartman
Subscribe to our Awesome Newsletter.
Keep Learning
Here are some related guides and posts that you might enjoy next.
How To Have Deep Dive Neuromonitoring Conversations That Pays Off…
How To Have A Neuromonitoring Discussion One of the reasons for starting this website was to make sure I was part of the neuromonitoring conversation. It was a decision I made early in my career... and I'm glad I did. Hearing the different perspectives and experiences...
Intraoperative EMG: Referential or Bipolar?
Recording Electrodes For EMG in the Operating Room: Referential or Bipolar? If your IONM manager walked into the OR in the middle of your case, took a look at your intraoperative EMG traces and started questioning your setup, could you defend yourself? I try to do...
BAER During MVD Surgery: A New Protocol?
BAER (Brainstem Auditory Evoked Potentials) During Microvascular Decompression Surgery You might remember when I was complaining about using ABR in the operating room and how to adjust the click polarity to help obtain a more reliable BAER. But my first gripe, having...
Bye-Bye Neuromonitoring Forum
Goodbye To The Neuromonitoring Forum One area of the website that I thought had the most potential to be an asset for the IONM community was the neuromonitoring forum. But it has been several months now and it is still a complete ghost town. I'm honestly not too...
EMG Nerve Monitoring During Minimally Invasive Fusion of the Sacroiliac Joint
Minimally Invasive Fusion of the Sacroiliac Joint Using EMG Nerve Monitoring EMG nerve monitoring in lumbar surgery makes up a large percentage of cases monitored every year. Using EMG nerve monitoring during SI joint fusions seems to be less utilized, even though the...
Physical Exam Scope Of Practice For The Surgical Neurophysiologist
SNP's Performing A Physical Exam: Who Should Do It And Who Shouldn't... Before any case is monitored, all pertinent patient history, signs, symptoms, physical exam findings and diagnostics should be gathered, documented and relayed to any oversight physician that may...
Help Spread The Word. Social Media Works!





0 Comments